N2 (UK) Ltd was first started in March 2013 by two director/owners of
the medical manufacturing facility Global Orthopaedics UK Ltd.
In 2013 it became apparent to us a dedicated company was required to deal solely with the veterinary industry, one that could dedicate time and money to research, development, and intellectual property protection, whilst Global Orthopaedics focused on maintaining its high quality and regulated manufacturing facility to both the veterinary and human markets. Thus N2 (UK) Ltd was born.
Neil Walters and Nick Gardener have both been at the forefront of medical human and veterinary orthopaedic device manufacture and design with over 30 years of experience between them.
Nick remains MD of Global Orthopaedics while also being heavily involved in N2’s design and marketing.
Neil is CEO of N2 UK Ltd ensuring the smooth running of the company, while also maintaining his role at Global Orthopaedics as Regulatory Affairs Director.
Having control of both facilities enables us to coordinate manufacture to sales demand and keep our distributors in stock with short lead times.
Quality
Medical Grade Materials
- Products are manufactured from certified medical grade materials
- Products are manufactured under an ISO 13485 quality management system
- Products are finished and laser identified
- Products are inspected to ISO:2859-1
- Products are cleaned and passivated ready for sterilisation
- Bioburden tests are carried out to ensure a low CFU (colony forming unit) count is maintained
Value
Fair Pricing Policy
- Having control of both facilities enables us to produce cost-effective batch runs and keep stock levels to an economic level
- Manufacturing under an ISO 13485 quality management system ensures that continual process improvements are achieved
- We are able to keep prices low through improved manufacturing times. Our aim is to continue this trend into the foreseeable future
- We believe in charging fair prices and not just replicating competitors prices.
Responsible
Reduced Carbon Footprint
- Implants are manufactured in the United Kingdom
- Raw materials are sourced and certified from Europe
- Transport of materials and finished goods are kept to a minimum
- Machining strategies and tool coatings help reduce the power requirements of machine tools
- Automation enables an additional 50% unmanned machining time reducing the impact from heat and lighting
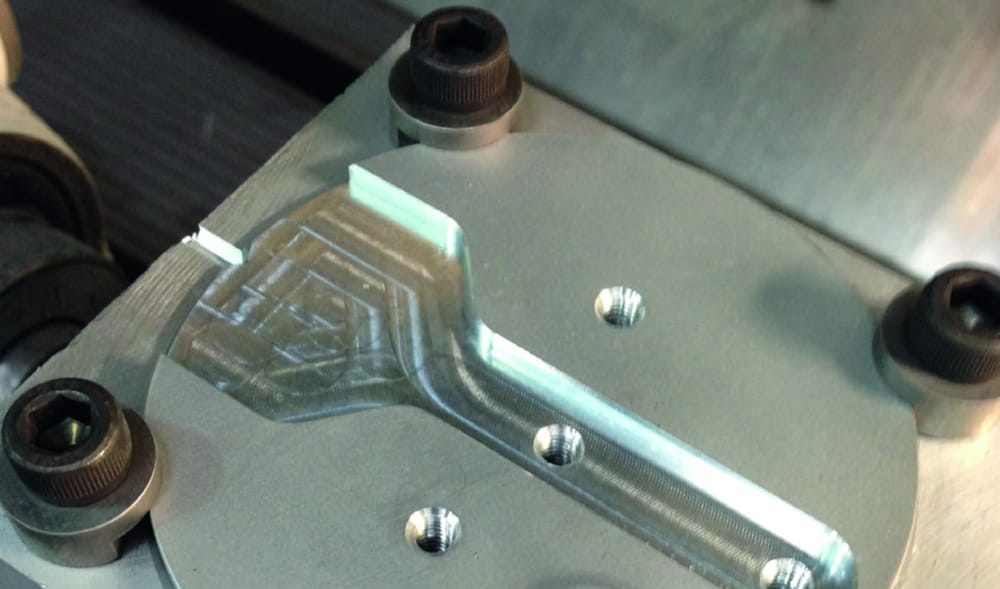
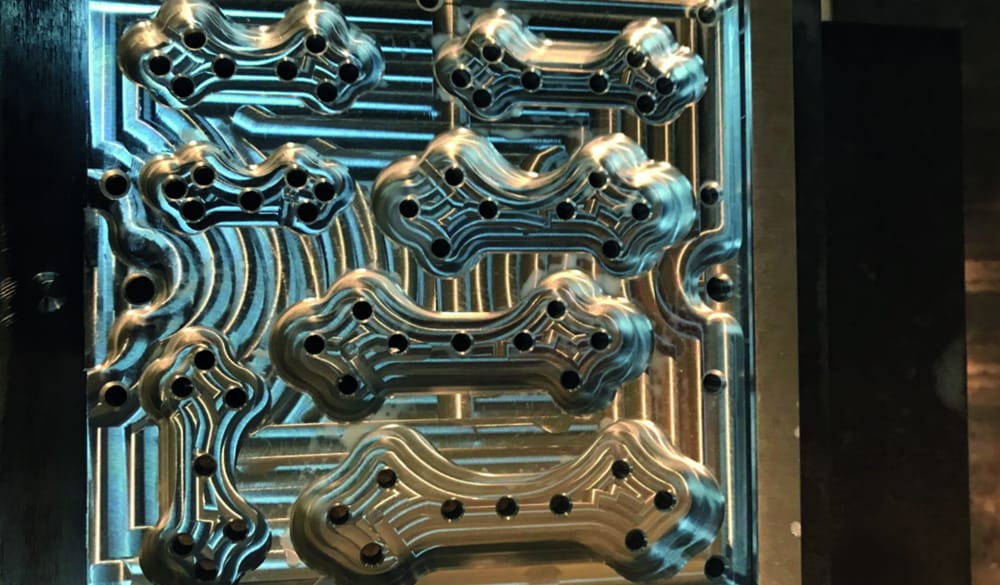
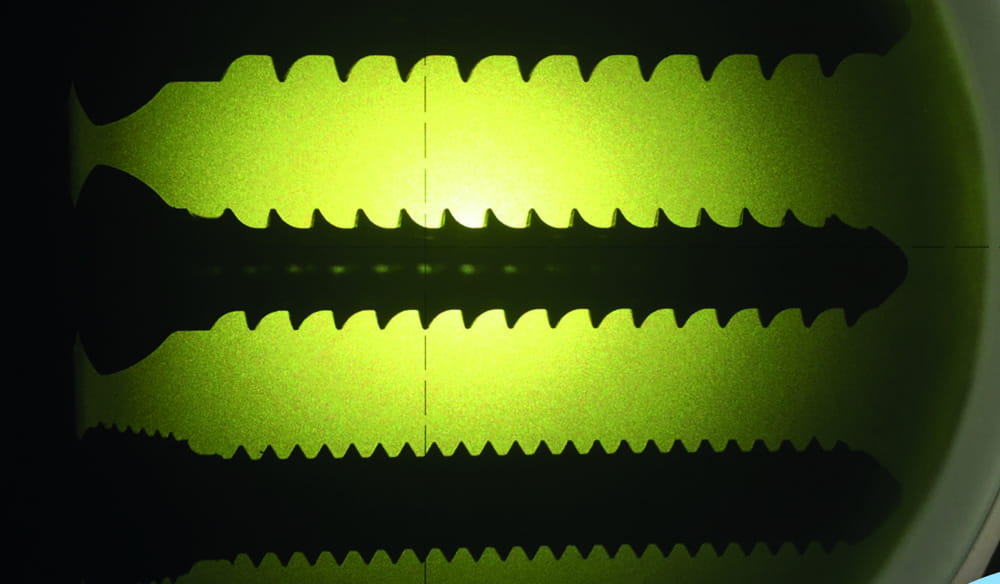
Manufacturing Facilities in the UK
We are proud of our complete manufacturing facilities in the UK. We would be more than happy to arrange a guided tour of the facilities to see how we manufacturer your implants and prove they are never ‘bought in’ or ‘reprocessed’ from afar.
- CAD/CAM
- Electropolishing
- Laser Marking
- CNC Milling, CNC Multi Axis Turning & Threading
- Vibratory Finishing including Diamond, Porceline and Ultrasonic
- Packaging Solutions